Editor’s Note: Every year, 40 or so students in the MIT Center for Transportation & Logistics' (MIT CTL) Master of Supply Chain Management (SCM) program complete one-year thesis research projects. The students are early-career business professionals from multiple countries with 2 to 10 years of experience in the industry. Most of the research projects are chosen, sponsored by, and carried out in collaboration with multinational corporations. Joint teams that include MIT SCM students and MIT CTL faculty work on the real-world problems. In this series, we summarize a selection of the latest SCM research.
The SCM thesis Serialization of Prescription Drugs in the US: A Centralized View was authored by Aisha Nabiyeva and David Z. Y. Wu, and supervised by Dr. Bruce Arntzen, Executive Director, Supply Chain Management Program, MIT Center for Transportation & Logistics. For more information on the research please contact Bruce Arntzen at [email protected].
With the introduction of the Drug Supply Chain Security Act (DSCSA), all players within the pharmaceutical industry are confronting the challenge of implementing a serialized drug tracing system with little guidance on data standards, roles, or accountabilities.
The research sponsor companies, both players in the pharmaceutical industry, wanted to objectively determine the best way to achieve system-wide serialization. The best method would balance the costs of serialization against the importance of maintaining system reliability, resiliency and scalability.
To understand the full extent of possible implementation options, we identified two key decision variables; the Data Management Model (Centralized vs Decentralized), the Relational Model (Individual Unit-Level vs Nested Parent-Child). A combination of each of these variables was then used to determine the optimal serialization scenario.
Challenges of Serialization
Currently, the pharmaceutical industry uses tracking and tracing technology heavily in the upstream supply chain. Maintaining visibility of the source of Active Pharmaceutical Ingredients (API) is of utmost importance. Resilient lot and batch identification methods have long been used between supplier and manufacturer supply chains. However, serialization seeks to extend the identification of drugs down to the individual level, so that each unit may be traced back through the supply chain.
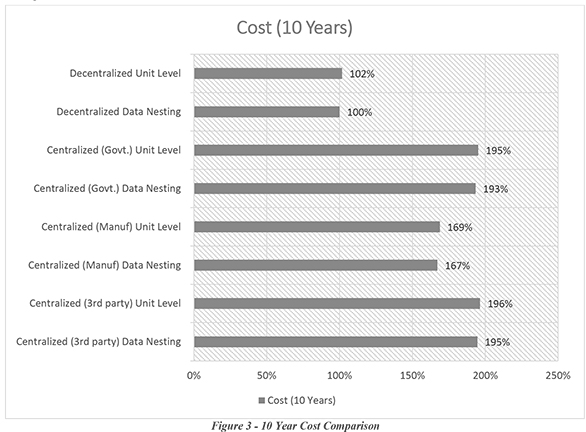
Although simple at first glance, the challenges of serialization lie in the implementation: Each of the individual scenarios identified offered advantages and disadvantages. A Centralized Data Management Model retains data integrity better, but comes at a higher cost; a unit-level Relational Model is easy to implement, but difficult to scale; External 3rd Party-Owned databases would be easier to build in theory, but are unproven in operation.
Evaluation of the scenarios took the form of a top-down analysis using publicly available industry information, which was used to build an initial cost model for each of the serialization scenarios. This was further validated by a bottom-up analysis with data provided by the thesis sponsor companies. Qualitative interviews with industry stakeholders provided further validation around which options were the most feasible.
Application and Next Steps
Through the top-down and bottom-up analysis, it was determined that a decentralized data management model would provide the lowest cost implementation, but that a centralized model would offer the best data integrity and database resiliency.
Key to successful pharmaceutical serialization is the availability of more detailed government specifications on data standards and accountabilities. Although it is possible to objectively determine an ideal solution, the constraints of real life will make any suggestions infeasible without government guidance. Alignment of systems, processes, and responsibilities need to be achieved before serialization moves from theory to reality.
The challenges of implementing serialization may extend to industries beyond pharmaceuticals. Although the drive behind this research was the introduction of the DSCSA, efforts in other industries to determine optimal track and trace solutions could follow a similar methodology to the one described.
SC
MR


Latest Supply Chain News
- How CPG brands can deliver on supplier diversity promises
- How S&OP provides the answer to in-demand products
- AI, virtual reality is bringing experiential learning into the modern age
- Humanoid robots’ place in an intralogistics smart robot strategy
- Tips for CIOs to overcome technology talent acquisition troubles
- More News
Latest Podcast

 Explore
Explore
The Academy News
- AI, virtual reality is bringing experiential learning into the modern age
- Predicting stockouts: Enhancing FMCG resilience through data-driven insights
- Finding the Right Approach for Supply Chain Education
- The Supply Chain Triad
- Innovating Supply Chain Higher Education with Generative AI
- How Smart Supply Chain Management Boosts Brand Identity
- More The Academy
Latest Academy Resources

Subscribe

Supply Chain Management Review delivers the best industry content.

Editors’ Picks